The SEQTEST Procedure
-
Overview

- Getting Started
-
Syntax

-
Details
 Input Data SetsBoundary VariablesInformation Level Adjustments at Future StagesBoundary Adjustments for Information LevelsBoundary Adjustments for Minimum Error SpendingBoundary Adjustments for Overlapping Lower and Upper beta BoundariesStochastic CurtailmentRepeated Confidence IntervalsAnalysis after a Sequential TestAvailable Sample Space Orderings in a Sequential TestApplicable Tests and Sample Size ComputationTable OutputODS Table NamesGraphics OutputODS Graphics
Input Data SetsBoundary VariablesInformation Level Adjustments at Future StagesBoundary Adjustments for Information LevelsBoundary Adjustments for Minimum Error SpendingBoundary Adjustments for Overlapping Lower and Upper beta BoundariesStochastic CurtailmentRepeated Confidence IntervalsAnalysis after a Sequential TestAvailable Sample Space Orderings in a Sequential TestApplicable Tests and Sample Size ComputationTable OutputODS Table NamesGraphics OutputODS Graphics -
Examples
 Testing the Difference between Two ProportionsTesting an Effect in a Regression ModelTesting an Effect with Early Stopping to Accept H0Testing a Binomial ProportionComparing Two Proportions with a Log Odds Ratio TestComparing Two Survival Distributions with a Log-Rank TestTesting an Effect in a Proportional Hazards Regression ModelTesting an Effect in a Logistic Regression ModelConducting a Trial with a Nonbinding Acceptance Boundary
Testing the Difference between Two ProportionsTesting an Effect in a Regression ModelTesting an Effect with Early Stopping to Accept H0Testing a Binomial ProportionComparing Two Proportions with a Log Odds Ratio TestComparing Two Survival Distributions with a Log-Rank TestTesting an Effect in a Proportional Hazards Regression ModelTesting an Effect in a Logistic Regression ModelConducting a Trial with a Nonbinding Acceptance Boundary - References
The SEQTEST procedure displays the “Design Information” and “Test Information” tables by default.
The “Conditional Power Information” table displays the following information under a hypothetical reference:
-
stopping stage
-
MLE, observed maximum likelihood estimate
-
conditional power under the hypothetical reference
For a one-sided test, the power are derived under hypothetical references ![]() and
and ![]() , where
, where ![]() is the observed statistic,
is the observed statistic, ![]() is the alternative reference, and
is the alternative reference, and ![]() are the values specified in the CREF= option. For a two-sided test, the power are derived under the hypothetical references
are the values specified in the CREF= option. For a two-sided test, the power are derived under the hypothetical references
![]() ,
, ![]() , and
, and ![]() , where
, where ![]() is the lower alternative reference and
is the lower alternative reference and ![]() is the upper alternative reference. The default is CREF= 0 0.5 1.0 1.5.
is the upper alternative reference. The default is CREF= 0 0.5 1.0 1.5.
The “Design Information” table displays the design specifications and derived statistics. The derived Max Information (Percent Fixed-Sample) is the maximum information for the sequential design in percentage of the corresponding fixed-sample information.
The Null Ref ASN (Percent Fixed-Sample) is the average sample size required under the null hypothesis for the group sequential design in percentage of the corresponding fixed-sample design. Similarly, the Alt Ref ASN (Percent Fixed-Sample) is the average sample size required under the alternative reference for the group sequential design in percentage of the corresponding fixed-sample design.
The “Error Spending Information” table displays the following information at each stage:
-
proportion of information
-
actual information level, if the maximum information is either specified or derived
-
cumulative error spending for each boundary
The “Parameter Estimates” table displays the following information at the conclusion of a sequential trial:
-
stopping stage
-
parameter estimate
-
median and confidence limits based on the specified ordering
-
p-value for the hypothesis
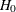 based on the specified ordering
based on the specified ordering
The “Powers and Expected Sample Sizes” table displays the following information under each of the specified hypothetical references ![]() , where
, where ![]() is the alternative reference and
is the alternative reference and ![]() are values specified in the CREF= option.
are values specified in the CREF= option.
-
coefficient
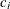 for the hypothetical references. The value
for the hypothetical references. The value 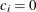 corresponds to the null hypothesis and
corresponds to the null hypothesis and 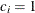 corresponds to the alternative hypothesis
corresponds to the alternative hypothesis
-
power
-
expected sample size, as percentage of fixed-sample size
For a one-sided design, the power and expected sample sizes under the hypothetical references ![]() are displayed.
are displayed.
For a two-sided symmetric design, the power and expected sample sizes under each of the hypothetical references ![]() are displayed, where
are displayed, where ![]() is the upper alternative reference.
is the upper alternative reference.
For a two-sided asymmetric design, the power and expected sample sizes under each of the hypothetical references ![]() and
and ![]() are displayed, where
are displayed, where ![]() and
and ![]() are the lower and upper alternative references, respectively.
are the lower and upper alternative references, respectively.
For a two-sided design, the power is the probability of correctly rejecting the null hypothesis for the correct alternative.
Thus, under the null hypothesis, the displayed power corresponds to a one-sided Type I error probability level—that is, the
lower ![]() level or the upper
level or the upper ![]() level.
level.
The expected sample size as a percentage of the corresponding fixed-sample design is
where ![]() is the stopping probability at stage k,
is the stopping probability at stage k, ![]() is the expected information level, and
is the expected information level, and ![]() is the information level for the fixed-sample design.
is the information level for the fixed-sample design.
The “Predictive Power Information” table displays the following information:
-
stopping stage
-
MLE, observed maximum likelihood estimate
-
predictive power
The “Repeated Confidence Intervals” table displays the following information for the observed statistic at each stage:
-
information level
-
parameter estimate
-
rejection confidence limits. The null hypothesis is rejected for the upper alternative if the lower rejection confidence limit is greater than the null parameter value. Similarly, the null hypothesis is rejected for the lower alternative if the upper rejection confidence limit is less than the null parameter value.
-
acceptance confidence limits. The upper alternative hypothesis is rejected if the upper acceptance confidence limit is less than the upper alternative value. Similarly, the lower alternative hypothesis is rejected if the lower acceptance confidence limit is greater than the upper alternative value. For a two-sided design, if both upper and lower alternative hypothesis are rejected, the null hypothesis is accepted.
The “Expected Cumulative Stopping Probabilities” table displays the following information under each of the specified hypothetical references ![]() , where
, where ![]() are values specified in the CREF= option, and
are values specified in the CREF= option, and ![]() is the alternative reference:
is the alternative reference:
-
coefficient
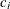 for the hypothetical references. The value
for the hypothetical references. The value 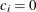 corresponds to the null hypothesis, and
corresponds to the null hypothesis, and 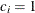 corresponds to the alternative hypothesis
corresponds to the alternative hypothesis
-
expected stopping stage
-
source of the stopping probability: reject
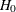 (with STOP=REJECT or STOP=BOTH), accept
(with STOP=REJECT or STOP=BOTH), accept 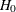 (with STOP=ACCEPT or STOP=BOTH), or either reject or accept
(with STOP=ACCEPT or STOP=BOTH), or either reject or accept 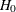 (with STOP=BOTH)
(with STOP=BOTH)
-
expected cumulative stopping probabilities at each stage
For a one-sided design, the expected cumulative stopping probabilities under the hypothetical references ![]() are displayed.
are displayed.
For a two-sided design, the expected cumulative stopping probabilities under each of the hypothetical references ![]() and
and ![]() are displayed, where
are displayed, where ![]() and
and ![]() are the lower and upper alternative references, respectively.
are the lower and upper alternative references, respectively.
Note that for a symmetric two-sided design, only the expected cumulative stopping probabilities under the hypothetical references
![]() are derived.
are derived.
The expected stopping stage is given by ![]() and is derived from the expected information level
and is derived from the expected information level
where ![]() is the stopping probability at stage k and
is the stopping probability at stage k and ![]() .
.
For equally spaced information levels, the expected stopping stage is reduced to the weighted average
The “Test Information” table displays the following information at each stage:
-
proportion of information
-
actual information level, if the maximum information is available from the input BOUNDARY= data set
-
alternative references with the specified statistic scale. If a p-value scale is specified, the standardized Z scale is used.
-
boundary values with the specified statistic scale to reject or accept the null hypothesis
Note that implicitly, the test information table also contains variables for the boundary scale, stopping criterion, and type
of alternative hypothesis. That is, if an ODS statement is used to save the table, the data set also contains the variables
_Scale_ for the boundary scale, _Stop_ for the stopping criterion, and _ALT_ for the type of alternative hypothesis.
If the test variable is specified, the table also displays the following:
-
test statistic
-
resulting action of test statistic: continue to the next stage, accept the null hypothesis
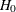 , or reject
, or reject