The SEQTEST Procedure
-
Overview

- Getting Started
-
Syntax

-
Details
 Input Data SetsBoundary VariablesInformation Level Adjustments at Future StagesBoundary Adjustments for Information LevelsBoundary Adjustments for Minimum Error SpendingBoundary Adjustments for Overlapping Lower and Upper beta BoundariesStochastic CurtailmentRepeated Confidence IntervalsAnalysis after a Sequential TestAvailable Sample Space Orderings in a Sequential TestApplicable Tests and Sample Size ComputationTable OutputODS Table NamesGraphics OutputODS Graphics
Input Data SetsBoundary VariablesInformation Level Adjustments at Future StagesBoundary Adjustments for Information LevelsBoundary Adjustments for Minimum Error SpendingBoundary Adjustments for Overlapping Lower and Upper beta BoundariesStochastic CurtailmentRepeated Confidence IntervalsAnalysis after a Sequential TestAvailable Sample Space Orderings in a Sequential TestApplicable Tests and Sample Size ComputationTable OutputODS Table NamesGraphics OutputODS Graphics -
Examples
 Testing the Difference between Two ProportionsTesting an Effect in a Regression ModelTesting an Effect with Early Stopping to Accept H0Testing a Binomial ProportionComparing Two Proportions with a Log Odds Ratio TestComparing Two Survival Distributions with a Log-Rank TestTesting an Effect in a Proportional Hazards Regression ModelTesting an Effect in a Logistic Regression ModelConducting a Trial with a Nonbinding Acceptance Boundary
Testing the Difference between Two ProportionsTesting an Effect in a Regression ModelTesting an Effect with Early Stopping to Accept H0Testing a Binomial ProportionComparing Two Proportions with a Log Odds Ratio TestComparing Two Survival Distributions with a Log-Rank TestTesting an Effect in a Proportional Hazards Regression ModelTesting an Effect in a Logistic Regression ModelConducting a Trial with a Nonbinding Acceptance Boundary - References
This example compares two survival distributions for the treatment effect. The example uses a power family method to generate two-sided asymmetric boundaries and then uses a proportional hazards regression model to test the hypothesis with a covariate.
A clinic is conducting a clinical study for the effect of a new cancer treatment. The study consists of mice exposed to a carcinogen and randomized to either the control group or the treatment group. The event of interest is the death from cancer induced by the carcinogen, and the response is the time from randomization to death.
Consider the proportional hazards regression model
where ![]() is an arbitrary and unspecified baseline hazard function,
is an arbitrary and unspecified baseline hazard function, TrtGp is the grouping variable for the two groups, Wgt is the initial weight of the mice, and ![]() and
and ![]() are the regression parameters associated with the variables
are the regression parameters associated with the variables TrtGp and Wgt, respectively. The grouping variable has the value 0 for each mouse in the control group and the value 1 for each mouse in
the treatment group.
The hypothesis ![]() with an alternative hypothesis
with an alternative hypothesis ![]() is used for the study.
is used for the study.
Suppose that from past experience, the median survival time for the control group is ![]() weeks. The study would like to detect a
weeks. The study would like to detect a ![]() weeks median survival time with a 80% power in the trial. Assuming exponential survival functions for the two groups, the
hazard rates can be computed from
weeks median survival time with a 80% power in the trial. Assuming exponential survival functions for the two groups, the
hazard rates can be computed from
where ![]() .
.
Thus, with the hazard rates ![]() and
and ![]() , the hazard ratio
, the hazard ratio ![]() and the alternative hypothesis
and the alternative hypothesis
Following the derivations in the section “Test for a Parameter in the Proportional Hazards Regression Model” in the chapter “The SEQDESIGN Procedure,” the required number of events for testing a parameter in ![]() is given by
is given by
where ![]() is the variance of
is the variance of TrtGp and ![]() is the proportion of variance of
is the proportion of variance of TrtGp explained by the variable Wgt.
If the two groups have the same number of mice in the study, then the MLE of the variance is ![]() . Further, if
. Further, if ![]() , then you can specify the MODEL=PHREG( XVARIANCE=0.25 XRSQUARE=0.10) option in the SAMPLESIZE statement in the SEQDESIGN
procedure to compute the required number of events and the individual number of events at each stage.
, then you can specify the MODEL=PHREG( XVARIANCE=0.25 XRSQUARE=0.10) option in the SAMPLESIZE statement in the SEQDESIGN
procedure to compute the required number of events and the individual number of events at each stage.
The following statements invoke the SEQDESIGN procedure and request a four-stage group sequential design for normally distributed
data. The design uses a two-sided alternative hypothesis with early stopping to reject the null hypothesis ![]() . A power family method is used to derive the boundaries.
. A power family method is used to derive the boundaries.
ods graphics on;
proc seqdesign altref=0.69315;
TwoSidedPowerFamily: design method=pow
nstages=4
alpha=0.075(lower=0.025)
beta=0.20;
samplesize model=phreg( xvariance=0.25 xrsquare=0.10
hazard=0.02451 accrate=10);
run;
ods graphics off;
The ALPHA=0.075(LOWER=0.025) option specifies a lower ![]() level 0.025 for the lower rejection boundary and an upper
level 0.025 for the lower rejection boundary and an upper ![]() level
level ![]() for the upper rejection boundary. The geometric average hazard
for the upper rejection boundary. The geometric average hazard ![]() is used in the HAZARD= option in the SAMPLESIZE statement to compute the required sample size. The specified ACCRATE=10 option
indicates that 10 mice will be accrued each week and the resulting minimum and maximum accrual times will be displayed.
is used in the HAZARD= option in the SAMPLESIZE statement to compute the required sample size. The specified ACCRATE=10 option
indicates that 10 mice will be accrued each week and the resulting minimum and maximum accrual times will be displayed.
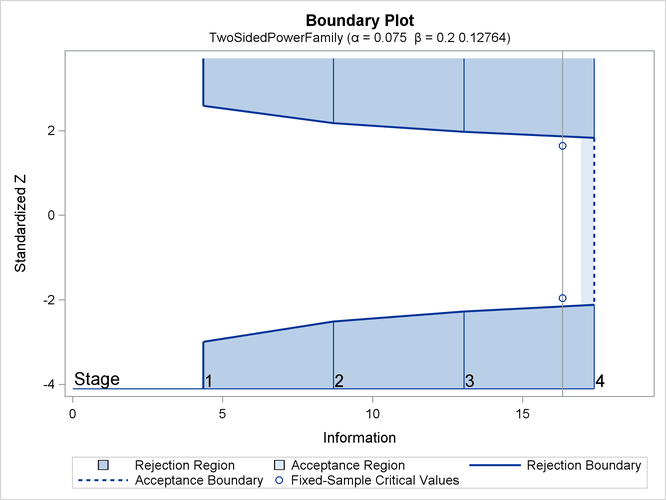
The “Design Information” table in Output 88.7.1 displays the design specifications and the derived statistics.
Output 88.7.1: Design Information
| Design Information | |
|---|---|
| Statistic Distribution | Normal |
| Boundary Scale | Standardized Z |
| Alternative Hypothesis | Two-Sided |
| Early Stop | Reject Null |
| Method | Power Family |
| Boundary Key | Both |
| Alternative Reference | 0.69315 |
| Number of Stages | 4 |
| Alpha | 0.075 |
| Alpha (Lower) | 0.025 |
| Alpha (Upper) | 0.05 |
| Beta (Lower) | 0.2 |
| Beta (Upper) | 0.12764 |
| Power (Lower) | 0.8 |
| Power (Upper) | 0.87236 |
| Max Information (Percent of Fixed Sample) | 106.468 |
| Max Information | 17.39288 |
| Null Ref ASN (Percent of Fixed Sample) | 104.3691 |
| Lower Alt Ref ASN (Number of Events) | 58.04014 |
| Upper Alt Ref ASN (Number of Events) | 52.05395 |
The “Boundary Information” table in Output 88.7.2 displays the information level, alternative reference, and boundary values at each stage. By default (or equivalently if you specify BOUNDARYSCALE=STDZ), the procedure displays the output boundaries with the standardized Z statistic.
Output 88.7.2: Boundary Information
| Boundary Information (Standardized Z Scale) Null Reference = 0 |
|||||||
|---|---|---|---|---|---|---|---|
| _Stage_ | Alternative | Boundary Values | |||||
| Information Level | Reference | Lower | Upper | ||||
| Proportion | Actual | Events | Lower | Upper | Alpha | Alpha | |
| 1 | 0.2500 | 4.348221 | 19.32543 | -1.44538 | 1.44538 | -2.98871 | 2.59149 |
| 2 | 0.5000 | 8.696441 | 38.65085 | -2.04408 | 2.04408 | -2.51320 | 2.17917 |
| 3 | 0.7500 | 13.04466 | 57.97628 | -2.50348 | 2.50348 | -2.27093 | 1.96910 |
| 4 | 1.0000 | 17.39288 | 77.3017 | -2.89077 | 2.89077 | -2.11334 | 1.83246 |
With ODS Graphics enabled, a detailed boundary plot with the rejection and acceptance regions is displayed, as shown in Output 88.7.3.
With the MODEL=PHREG option in the SAMPLESIZE statement, the “Sample Size Summary” table in Output 88.7.4 displays the parameters used in the sample size computation for the proportional hazards regression model.
Output 88.7.4: Required Sample Size Summary
| Sample Size Summary | |
|---|---|
| Test | PH Reg Parameter |
| Parameter | 0.69315 |
| X Variance | 0.25 |
| R Square (X) | 0.1 |
| Hazard Rate | 0.02451 |
| Accrual | Uniform |
| Accrual Rate | 10 |
| Min Accrual Time | 7.73017 |
| Min Sample Size | 77.3017 |
| Max Accrual Time | 27.97872 |
| Max Sample Size | 279.7872 |
| Max Number of Events | 77.3017 |
With a minimum accrual time of 7.73 weeks and maximum accrual time of 27.98 weeks, an accrual time of 20 weeks is used in the study. The “Numbers of Events” table in Output 88.7.5 displays the required numbers of events for the group sequential clinical trial.
Output 88.7.5: Required Sample Sizes
| Numbers of Events (D) Z Test for PH Regression Parameter |
||
|---|---|---|
| _Stage_ | D | Information |
| 1 | 19.33 | 4.3482 |
| 2 | 38.65 | 8.6964 |
| 3 | 57.98 | 13.0447 |
| 4 | 77.30 | 17.3929 |
The following statements invoke the SEQDESIGN procedure and provide more detailed sample size information with a 20-week accrual time:
proc seqdesign altref=0.69315;
TwoSidedPowerFamily: design method=pow
nstages=4
alpha=0.075(lower=0.025)
beta=0.20;
samplesize model=phreg( xvariance=0.25 xrsquare=0.10
hazard=0.02451
accrate=10 acctime=20);
ods output Boundary=Bnd_Time;
run;
The ODS OUTPUT statement with the BOUNDARY=BND_TIME option creates an output data set named BND_TIME which contains the resulting boundary information for the subsequent sequential tests.
With an accrual time of 20 weeks, the “Sample Size Summary” table in Output 88.7.6 displays the follow-up time for the trial.
Output 88.7.6: Sample Size Summary
| Sample Size Summary | |
|---|---|
| Test | PH Reg Parameter |
| Parameter | 0.69315 |
| X Variance | 0.25 |
| R Square (X) | 0.1 |
| Hazard Rate | 0.02451 |
| Accrual | Uniform |
| Accrual Rate | 10 |
| Accrual Time | 20 |
| Follow-up Time | 10.34195 |
| Total Time | 30.34195 |
| Max Number of Events | 77.3017 |
| Max Sample Size | 200 |
| Expected Sample Size (Null Ref) | 199.4282 |
| Expected Sample Size (Alt Ref) | 188.6561 |
| Follow-up Time (Ceiling Time) | 11 |
| Total Time (Ceiling Time) | 31 |
The “Numbers of Events and Sample Sizes” table in Output 88.7.7 displays the required sample sizes for the group sequential clinical trial.
Output 88.7.7: Numbers of Events and Sample Sizes
| Numbers of Events (D) and Sample Sizes (N) Z Test for PH Regression Parameter |
||||||||
|---|---|---|---|---|---|---|---|---|
| _Stage_ | Fractional Time | Ceiling Time | ||||||
| D | Time | N | Information | D | Time | N | Information | |
| 1 | 19.33 | 13.2362 | 132.36 | 4.3482 | 21.49 | 14 | 140.00 | 4.8359 |
| 2 | 38.65 | 19.1466 | 191.47 | 8.6964 | 41.90 | 20 | 200.00 | 9.4281 |
| 3 | 57.98 | 24.3744 | 200.00 | 13.0447 | 60.14 | 25 | 200.00 | 13.5309 |
| 4 | 77.30 | 30.3420 | 200.00 | 17.3929 | 79.26 | 31 | 200.00 | 17.8346 |
Thus, the study will perform three interim analyses after 14, 20, and 25 weeks and a final analysis after 31 weeks if the study does not stop at any of the interim analyses.
Suppose 140 mice are available for the first interim analysis after week 14. Output 88.7.8 lists the first 10 observations in the data set weeks_1.
Output 88.7.8: Clinical Trial Data
| First 10 Obs in the Trial Data |
| Obs | TrtGp | Event | Wgt | Weeks |
|---|---|---|---|---|
| 1 | 0 | 0 | 22.1659 | 12 |
| 2 | 1 | 0 | 28.4458 | 12 |
| 3 | 0 | 0 | 26.2857 | 12 |
| 4 | 1 | 0 | 25.0283 | 12 |
| 5 | 0 | 0 | 21.5114 | 12 |
| 6 | 1 | 0 | 23.2240 | 12 |
| 7 | 0 | 1 | 22.6845 | 6 |
| 8 | 1 | 0 | 27.9292 | 12 |
| 9 | 0 | 0 | 22.5514 | 12 |
| 10 | 1 | 1 | 27.3793 | 11 |
The TrtGp variable is a grouping variable with the value 0 for a mouse in the placebo control group and the value 1 for a mouse in
the treatment group.
The Weeks variable is the survival time variable measured in weeks and the Event variable is the censoring variable with the value 0 indicating censoring. That is, the values of Weeks are considered censored if the corresponding values of Event are 0; otherwise, they are considered as event times.
The following statements use the PHREG procedure to estimate the treatment effect after adjusting for the Wgt variable at stage 1:
proc phreg data=Time_1; model Weeks*Event(0)= TrtGp Wgt; ods output parameterestimates=Parms_Time1; run;
The following statements create and display (in Output 88.7.9) the data set for the treatment effect MLE statistic and its associated standard error. Note that for a MLE statistic, the inverse of the variance of the statistic is the information.
data Parms_Time1; set Parms_Time1; if Parameter='TrtGp'; _Scale_='MLE'; _Stage_= 1; keep _Scale_ _Stage_ Parameter Estimate StdErr; run; proc print data=Parms_Time1; title 'Statistics Computed at Stage 1'; run;
Output 88.7.9: Statistics Computed at Stage 1
| Statistics Computed at Stage 1 |
| Obs | Parameter | Estimate | StdErr | _Scale_ | _Stage_ |
|---|---|---|---|---|---|
| 1 | TrtGp | 0.00836 | 0.46588 | MLE | 1 |
The following statements invoke the SEQTEST procedure to test for early stopping at stage 1:
ods graphics on;
proc seqtest Boundary=Bnd_Time
Parms(Testvar=TrtGp)=Parms_Time1
infoadj=prop
order=lr
;
ods output Test=Test_Time1;
run;
ods graphics off;
The BOUNDARY= option specifies the input data set that provides the boundary information for the trial at stage 1, which was
generated in the SEQDESIGN procedure. The PARMS=PARMS_TIME1 option specifies the input data set PARMS_TIME1 that contains the test statistic and its associated standard error at stage 1, and the TESTVAR=TRTGP option identifies the
test variable TRTGP in the data set.
If the computed information level for stage 1 is not the same as the value provided in the BOUNDARY= data set, the INFOADJ=PROP option (which is the default) proportionally adjusts the information levels at future interim stages from the levels provided in the BOUNDARY= data set. The ORDER=LR option uses the LR ordering to derive the p-value, the unbiased median estimate, and the confidence limits for the regression slope estimate.
The ODS OUTPUT statement with the TEST=TEST_TIME1 option creates an output data set named TEST_TIME1 which contains the updated boundary information for the test at stage 1. The data set also provides the boundary information
that is needed for the group sequential test at the next stage.
The “Design Information” table in Output 88.7.10 displays design specifications. By default (or equivalently if you specify BOUNDARYKEY=ALPHA), the boundary values are modified
for the new information levels to maintain the Type I ![]() level. The maximum information and the power have been modified for the new information levels.
level. The maximum information and the power have been modified for the new information levels.
Output 88.7.10: Design Information
| Design Information | |
|---|---|
| BOUNDARY Data Set | WORK.BND_TIME |
| Data Set | WORK.PARMS_TIME1 |
| Statistic Distribution | Normal |
| Boundary Scale | Standardized Z |
| Alternative Hypothesis | Two-Sided |
| Early Stop | Reject Null |
| Number of Stages | 4 |
| Alpha | 0.075 |
| Alpha (Lower) | 0.025 |
| Alpha (Upper) | 0.05 |
| Beta (Lower) | 0.20048 |
| Beta (Upper) | 0.12795 |
| Power (Lower) | 0.79952 |
| Power (Upper) | 0.87205 |
| Max Information (Percent of Fixed Sample) | 106.5982 |
| Max Information | 17.3928828 |
| Null Ref ASN (Percent of Fixed Sample) | 104.4715 |
| Lower Alt Ref ASN (Percent of Fixed Sample) | 79.7886 |
| Upper Alt Ref ASN (Percent of Fixed Sample) | 71.53877 |
The “Test Information” table in Output 88.7.11 displays the boundary values for the test statistic with the MLE statistic scale.
Output 88.7.11: Sequential Tests
| Test Information (Standardized Z Scale) Null Reference = 0 |
||||||||
|---|---|---|---|---|---|---|---|---|
| _Stage_ | Alternative | Boundary Values | Test | |||||
| Information Level | Reference | Lower | Upper | TrtGp | ||||
| Proportion | Actual | Lower | Upper | Alpha | Alpha | Estimate | Action | |
| 1 | 0.2649 | 4.607347 | -1.48783 | 1.48783 | -2.92457 | 2.54086 | 0.01795 | Continue |
| 2 | 0.5099 | 8.869192 | -2.06428 | 2.06428 | -2.50505 | 2.17290 | . | |
| 3 | 0.7550 | 13.13104 | -2.51175 | 2.51175 | -2.27093 | 1.96941 | . | |
| 4 | 1.0000 | 17.39288 | -2.89077 | 2.89077 | -2.11635 | 1.83531 | . | |
With the INFOADJ=PROP option (which is the default), the information levels at interim stages 2 and 3 are derived proportionally
from the information levels in the BOUNDARY= data set. At stage 1, the standardized Z statistic 0.01795 is between the lower and upper ![]() boundary values of –2.92457 and 2.54086, so the trial continues to the next stage.
boundary values of –2.92457 and 2.54086, so the trial continues to the next stage.
Note that the observed information level 4.6073 corresponds to a proportion of 0.2649 in the information level. If the observed information level is much larger than the target proportion of 0.25, then you can decrease the accrual rate, accrual time, or follow-up time to achieve target information levels for subsequent stages. These modifications should be specified in the study plan before the study begins.
With ODS Graphics enabled, a boundary plot with test statistics is displayed, as shown in Output 88.7.12. As expected, the test statistic is in the continuation region between the lower and upper ![]() boundary values.
boundary values.
The following statements use the PHREG procedure to compute the MLE statistic and its associated standard error at stage 2:
proc phreg data=Time_2; model Weeks*Event(0)= TrtGp Wgt; ods output parameterestimates= Parms_Time2; run;
The following statements create the data set for the MLE statistic and its associated standard error at stage 2:
data Parms_Time2; set Parms_Time2; if Parameter='TrtGp'; _Scale_='MLE'; _Stage_= 2; keep _Scale_ _Stage_ Parameter Estimate StdErr; run;
The following statements invoke the SEQTEST procedure to test for early stopping at stage 2:
proc seqtest Boundary=Test_Time1
Parms(Testvar=TrtGp)=Parms_Time2
infoadj=prop
order=lr
;
ods output Test=Test_Time2;
run;
The BOUNDARY= option specifies the input data set that provides the boundary information for the trial at stage 2, which was generated by the SEQTEST procedure at the previous stage. The PARMS= option specifies the input data set that contains the test statistic and its associated standard error at stage 2, and the TESTVAR= option identifies the test variable in the data set.
The ODS OUTPUT statement with the TEST=TEST_TIME2 option creates an output data set named TEST_TIME2 which contains the updated boundary information for the test at stage 2. The data set also provides the boundary information
that is needed for the group sequential test at the next stage.
The “Test Information” table in Output 88.7.13 displays the boundary values for the test statistic with the MLE statistic scale. At stage 2, the standardized Z statistic –0.43552 is between the lower ![]() and upper boundary values, –2.47689 and 2.14819, respectively, so the trial continues to the next stage.
and upper boundary values, –2.47689 and 2.14819, respectively, so the trial continues to the next stage.
Output 88.7.13: Sequential Tests
| Test Information (Standardized Z Scale) Null Reference = 0 |
||||||||
|---|---|---|---|---|---|---|---|---|
| _Stage_ | Alternative | Boundary Values | Test | |||||
| Information Level | Reference | Lower | Upper | TrtGp | ||||
| Proportion | Actual | Lower | Upper | Alpha | Alpha | Estimate | Action | |
| 1 | 0.2649 | 4.607347 | -1.48783 | 1.48783 | -2.92457 | 2.54086 | 0.01795 | Continue |
| 2 | 0.5251 | 9.132918 | -2.09475 | 2.09475 | -2.47689 | 2.14819 | -0.43552 | Continue |
| 3 | 0.7625 | 13.2629 | -2.52433 | 2.52433 | -2.26878 | 1.96770 | . | |
| 4 | 1.0000 | 17.39288 | -2.89077 | 2.89077 | -2.12017 | 1.83880 | . | |
Since the data set PARMS_Time2 contains the test information only at stage 2, the information level at stage 1 in the TEST_Time1 data set is used to generate boundary values for the test.
Similarly, the test statistic at stage 3 is also between its corresponding lower and upper ![]() boundary values. The trial continues to the next stage.
boundary values. The trial continues to the next stage.
The following statements use the PHREG procedure to compute the MLE statistic and its associated standard error at the final stage:
proc phreg data=Time_4; model Weeks*Event(0)= TrtGp Wgt; ods output parameterestimates= Parms_Time4; run;
The following statements create and display (in Output 88.7.14) the data set for the MLE statistic and its associated standard error at each stage of the study:
data Parms_Time4; set Parms_Time4; if Parameter='TrtGp'; _Scale_='MLE'; _Stage_= 4; keep _Scale_ _Stage_ Parameter Estimate StdErr; run;
proc print data=Parms_Time4; title 'Statistics Computed at Stage 4'; run;
Output 88.7.14: Statistics Computed at Stage 4
| Statistics Computed at Stage 4 |
| Obs | Parameter | Estimate | StdErr | _Scale_ | _Stage_ |
|---|---|---|---|---|---|
| 1 | TrtGp | -0.04451 | 0.23971 | MLE | 4 |
The following statements invoke the SEQTEST procedure to test the hypothesis at stage 4:
ods graphics on;
proc seqtest Boundary=Test_Time3
Parms(Testvar=TrtGp)=Parms_Time4
order=lr
;
run;
ods graphics off;
The BOUNDARY= option specifies the input data set that provides the boundary information for the trial at stage 4, which was generated by the SEQTEST procedure at the previous stage. The PARMS= option specifies the input data set that contains the test statistic and its associated standard error at stage 4, and the TESTVAR= option identifies the test variable in the data set.
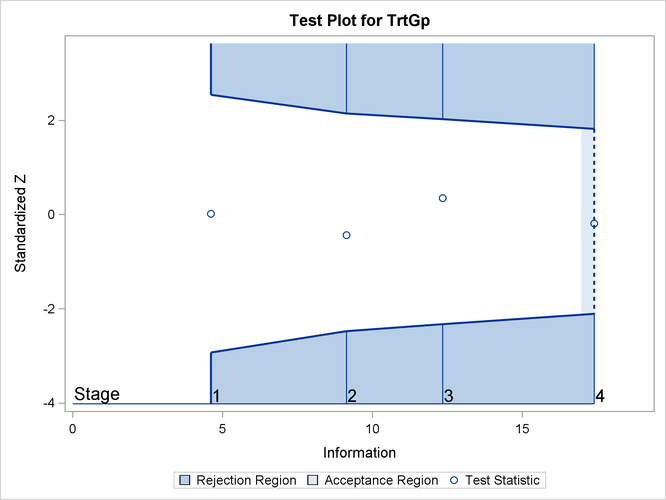
The “Test Information” table in Output 88.7.15 displays the boundary values for the test statistic. The standardized test statistic –0.1857 is between the lower and upper
![]() boundary values of –2.10447 and 1.82112, respectively, so the study stops and accepts the null hypothesis. That is, there
is no evidence of reduction in hazard rate for the new treatment.
boundary values of –2.10447 and 1.82112, respectively, so the study stops and accepts the null hypothesis. That is, there
is no evidence of reduction in hazard rate for the new treatment.
Output 88.7.15: Sequential Tests
| Test Information (Standardized Z Scale) Null Reference = 0 |
||||||||
|---|---|---|---|---|---|---|---|---|
| _Stage_ | Alternative | Boundary Values | Test | |||||
| Information Level | Reference | Lower | Upper | TrtGp | ||||
| Proportion | Actual | Lower | Upper | Alpha | Alpha | Estimate | Action | |
| 1 | 0.2647 | 4.607347 | -1.48783 | 1.48783 | -2.92457 | 2.54086 | 0.01795 | Continue |
| 2 | 0.5248 | 9.132918 | -2.09475 | 2.09475 | -2.47689 | 2.14819 | -0.43552 | Continue |
| 3 | 0.7095 | 12.34753 | -2.43566 | 2.43566 | -2.32705 | 2.02634 | 0.34864 | Continue |
| 4 | 1.0000 | 17.40274 | -2.89159 | 2.89159 | -2.10447 | 1.82112 | -0.18570 | Accept Null |
The “Test Plot” displays boundary values of the design and the test statistic at the first two stages, as shown in Output 88.7.16. It also shows that the test statistic is in the “Acceptance Region” between the lower and upper ![]() boundary values at stage 4.
boundary values at stage 4.
After the stopping of a trial, the “Parameter Estimates” table in Output 88.7.17 displays the stopping stage, parameter estimate, unbiased median estimate, confidence limits, and p-value under the null hypothesis ![]() .
.
Output 88.7.17: Parameter Estimates
| Parameter Estimates LR Ordering |
||||||
|---|---|---|---|---|---|---|
| Parameter | Stopping Stage |
MLE | p-Value for H0:Parm=0 |
Median Estimate |
95% Confidence Limits | |
| TrtGp | 4 | -0.044514 | 0.8525 | -0.044577 | -0.51461 | 0.42538 |
As expected, the two-sided p-value 0.8525 is not significant at the lower ![]() level and the upper
level and the upper ![]() level, and the two-sided 95% confidence interval contains the null value zero. The p-value, unbiased median estimate, and lower confidence limit depend on the ordering of the sample space
level, and the two-sided 95% confidence interval contains the null value zero. The p-value, unbiased median estimate, and lower confidence limit depend on the ordering of the sample space ![]() , where k is the stage number and z is the standardized Z statistic. With the specified LR ordering, the two-sided p-value is derived from the one-sided p-value
, where k is the stage number and z is the standardized Z statistic. With the specified LR ordering, the two-sided p-value is derived from the one-sided p-value
where ![]() is the observed test statistic at stage 4,
is the observed test statistic at stage 4, ![]() is a standardized normal variate at stage k, and
is a standardized normal variate at stage k, and ![]() and
and ![]() are the stage k lower and upper rejection boundary values, respectively.
are the stage k lower and upper rejection boundary values, respectively.
Thus,
where ![]() is the upper
is the upper ![]() level and
level and ![]() .
.
Since ![]() ,
, ![]() , which is greater than 0.50. Thus, the two-sided p-value is given by
, which is greater than 0.50. Thus, the two-sided p-value is given by ![]() .
.